Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »
- How Much Oxygen In Grams Will Form From 60 2 Mg Of N2o5 2n205 S 4no2 G O2 G 2 89 X 10 3 90 1 (27.32 KiB) Viewed 8 times
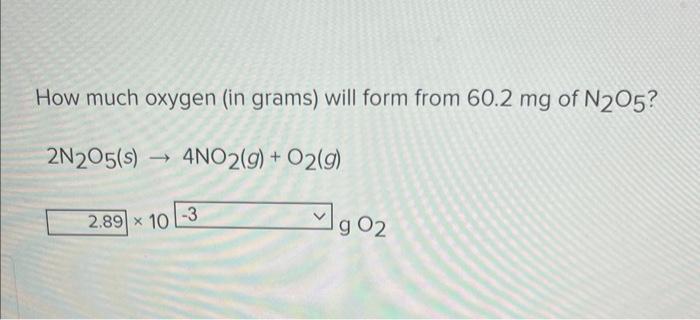
How much oxygen (in grams) will form from 60.2 mg of N2O5? 2N205(s)- - 4NO2(g) + O2(g) 2.89 x 10 -3 90₂
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!