Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »
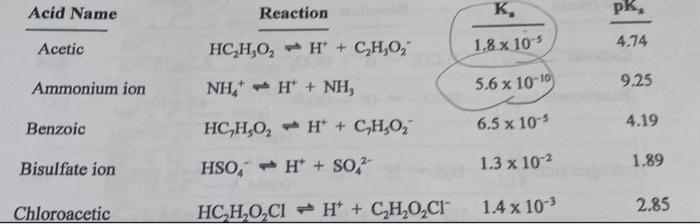
- Acid Name Reaction K Pk Acetic Hc H O H C H O 4 74 1 8 X 105 9 25 Ammonium Ion 5 6 X 10 10 Nh H Nh3 Benzoic Hc 1 (19.68 KiB) Viewed 12 times
- Acid Name Reaction K Pk Acetic Hc H O H C H O 4 74 1 8 X 105 9 25 Ammonium Ion 5 6 X 10 10 Nh H Nh3 Benzoic Hc 2 (18.35 KiB) Viewed 12 times
Acid Name Reaction K, pk, Acetic HC,H,O, H + C,H,O, 4.74 1.8 x 105 9.25 Ammonium ion 5.6 x 10-10 NH, H+ + NH3 Benzoic HC,H,O, HỒ + C,H,O, 6.5 x 10-5 4.19 1.89 Bisulfate ion 1.3 x 10-2 HSO, H* + SO,2- Chloroacetic HC,H,O,Cl + H + CH202C1 2.85 1.4 x 10-3
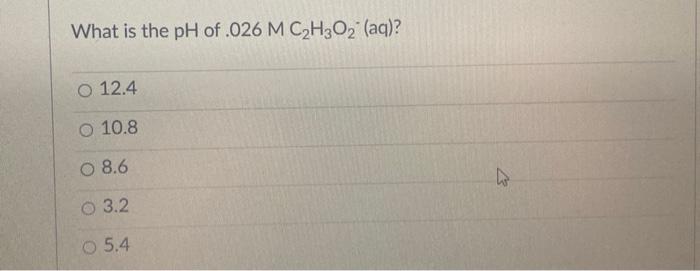
What is the pH of .026 M C H2O2 (aq)? O 12.4 O 10.8 O 8.6 O 3.2 O 5.4
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!