Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »
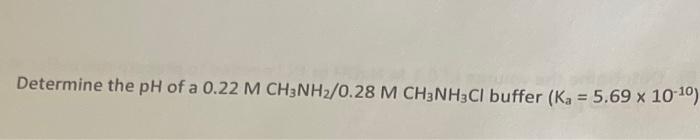
- Determine The Ph Of A 0 22 M Ch3nh2 0 28 M Ch3nh2cl Buffer Ka 5 69 X 10 10 1 (7.68 KiB) Viewed 7 times
Determine the pH of a 0.22 M CH3NH2/0.28 M CH3NH2Cl buffer (Ka = 5.69 x 10-10)
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!