Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »

- Calculate The Ph Of Each Of The Following Solutions Ka And K Values Are Given In Appendix D In The Textbook 9 3x10 1 (10.71 KiB) Viewed 29 times
- Calculate The Ph Of Each Of The Following Solutions Ka And K Values Are Given In Appendix D In The Textbook 9 3x10 2 (31.21 KiB) Viewed 29 times
- Calculate The Ph Of Each Of The Following Solutions Ka And K Values Are Given In Appendix D In The Textbook 9 3x10 3 (33.24 KiB) Viewed 29 times
- Calculate The Ph Of Each Of The Following Solutions Ka And K Values Are Given In Appendix D In The Textbook 9 3x10 4 (32.86 KiB) Viewed 29 times
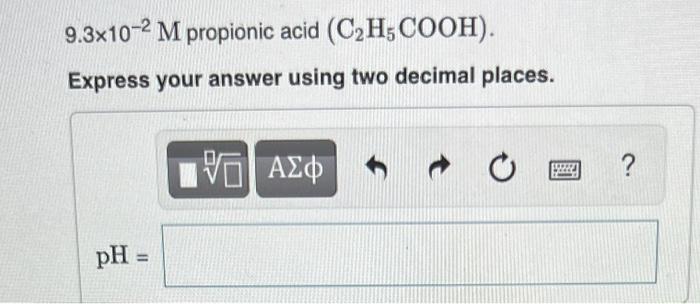
Calculate the pH of each of the following
solutions (Ka and K, values are given in Appendix D in the textbook).
9.3x10-2 M propionic acid (C2H, COOH). Express your answer using two decimal places. pH = VE ΑΣΦ C ?
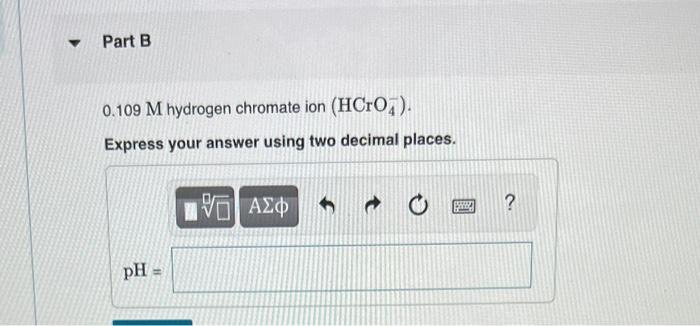
▼ Part B 0.109 M hydrogen chromate ion (HCrO). Express your answer using two decimal places. pH M 1951 ΑΣΦ S ?
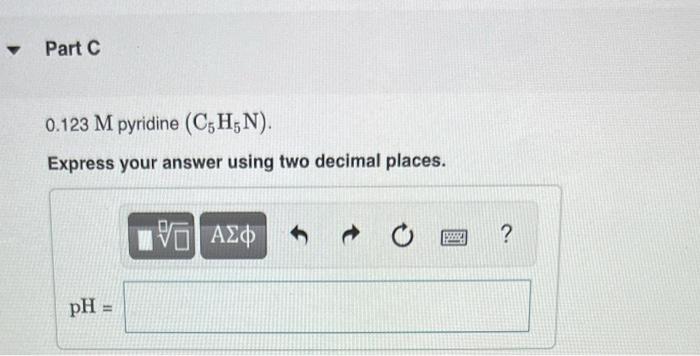
▼ Part C 0.123 M pyridine (C5H₁N). Express your answer using two decimal places. pH = VE ΑΣΦ 3 ?
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!