Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 1 (32.16 KiB) Viewed 12 times
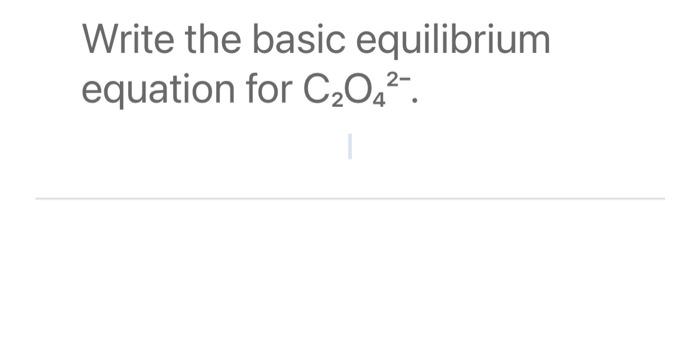
- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 2 (12.02 KiB) Viewed 12 times

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 3 (16.39 KiB) Viewed 12 times

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 4 (15.08 KiB) Viewed 12 times

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 5 (11.7 KiB) Viewed 12 times

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 6 (25.94 KiB) Viewed 12 times

- The Concentration Of Hydroxide Solution At 25 C Is 9 5 X 10 4 M What Is The Concentration Of The Hydronium Ion Ion In 7 (11.73 KiB) Viewed 12 times
The concentration of hydroxide solution at 25°C is 9.5 x 10-4 M. What is the concentration of the hydronium ion? ion in an aqueous
Write the basic equilibrium equation for C₂O4²-.
The pOH of an acidic solution is 10.89. What is [OH-]?
The pH of an acidic solution is 5.11. What is [OH-]?
What is the pH of a 6.1 x 10-6 M HBr solution?
What mass of LiOH would need to be dissolved in water to make 300.0 mL of a solution with a pH of 11.19?
What is the pH of a 6.1 x 10-6 M HBr solution?
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!