Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »
- The Atomic Mass Unit Equals 1 661 X 10 24 Grams What Is The Mass In Grams Of Each Molecule Of C6h1206 1 (15.2 KiB) Viewed 33 times
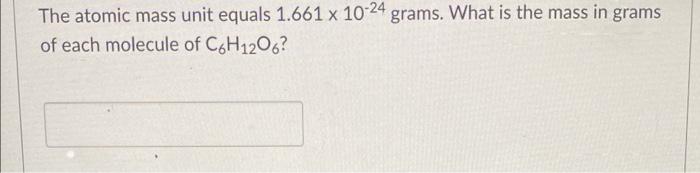
The atomic mass unit equals 1.661 x 10-24 grams. What is the mass in grams of each molecule of C6H1206?
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!