Business, Finance, Economics, Accounting, Operations Management, Computer Science, Electrical Engineering, Mechanical Engineering, Civil Engineering, Chemical Engineering, Algebra, Precalculus, Statistics and Probabilty, Advanced Math, Physics, Chemistry, Biology, Nursing, Psychology, Certifications, Tests, Prep, and more.
-
answerhappygod
- Site Admin
- Posts: 899603
- Joined: Mon Aug 02, 2021 8:13 am
Post
by answerhappygod »
PLEASE HELP!!
- Please Help 1 (35.57 KiB) Viewed 49 times
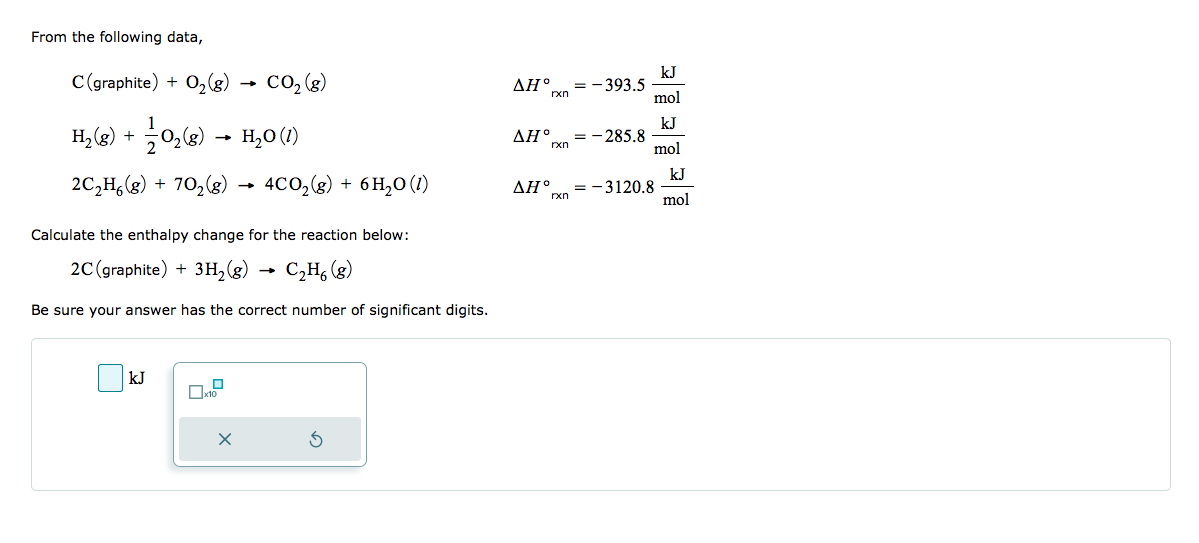
From the following data, C (graphite) + O₂(g) → CO₂ (8) H₂(g) + O₂(g) → H₂0 (1) 2C₂H6(g) + 70₂(g) → 4CO₂(g) + 6H₂O (1) Calculate the enthalpy change for the reaction below: 2C (graphite) + 3H₂(g) → C₂H6 (8) Be sure your answer has the correct number of significant digits. kJ X 3 ΔΗ° ΔΗ° ΔΗ° -393.5 = -285.8 rxn =-3120.8 rxn rxn kJ mol kJ mol = kJ mol
Join a community of subject matter experts. Register for FREE to view solutions, replies, and use search function. Request answer by replying!