Page 1 of 1
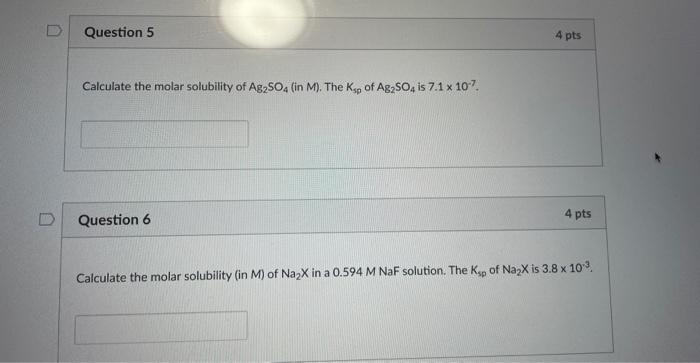
D Question 5 Calculate the molar solubility of Ag₂SO4 (in M). The Ksp of Ag2SO4 is 7.1 x 107. Question 6 4 pts 4 pts Cal
Posted: Wed Jul 06, 2022 11:00 am
by answerhappygod
- D Question 5 Calculate The Molar Solubility Of Ag So4 In M The Ksp Of Ag2so4 Is 7 1 X 107 Question 6 4 Pts 4 Pts Cal 1 (26.71 KiB) Viewed 17 times
D
Question 5 Calculate the molar solubility of Ag₂SO4 (in M). The Ksp of Ag2SO4 is 7.1 x 107.
Question 6 4 pts 4 pts Calculate the molar solubility (in M) of Na₂X in a 0.594 M NaF solution. The Ksp of Na₂x is 3.8 x 10¹³.