Page 1 of 1
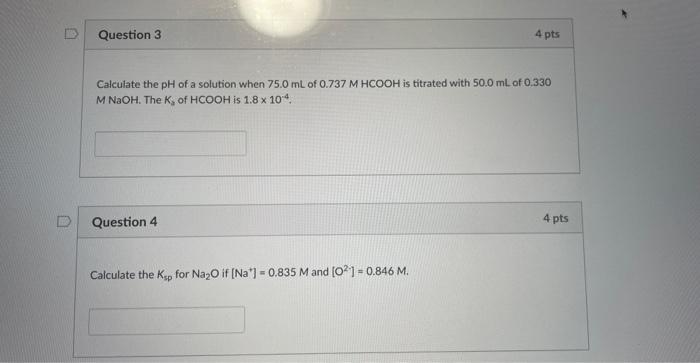
Question 3 Calculate the pH of a solution when 75.0 mL of 0.737 M HCOOH is titrated with 50.0 mL of 0.330 M NaOH. The K,
Posted: Wed Jul 06, 2022 11:00 am
by answerhappygod
- Question 3 Calculate The Ph Of A Solution When 75 0 Ml Of 0 737 M Hcooh Is Titrated With 50 0 Ml Of 0 330 M Naoh The K 1 (22.44 KiB) Viewed 12 times
Question 3 Calculate the pH of a solution when 75.0 mL of 0.737 M HCOOH is titrated with 50.0 mL of 0.330 M NaOH. The K, of HCOOH is 1.8 x 104.
Question 4 4 pts Calculate the Ksp for Na₂O if [Na] = 0.835 M and [021 - 0.846 M. 4 pts