Page 1 of 1
QUESTION 26 Determine the mass in grams of Al2(SO4)3 that contains 3.25 x 10^22 oxygen atoms.
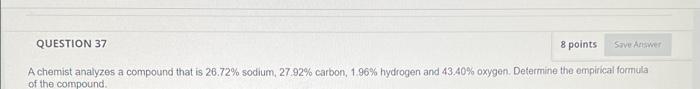
QUESTION 37 8 points Save
Posted: Wed Jul 06, 2022 10:55 am
by answerhappygod

- Question 26 Determine The Mass In Grams Of Al2 So4 3 That Contains 3 25 X 10 22 Oxygen Atoms Question 37 8 Points Save 1 (10.09 KiB) Viewed 12 times
- Question 26 Determine The Mass In Grams Of Al2 So4 3 That Contains 3 25 X 10 22 Oxygen Atoms Question 37 8 Points Save 2 (8.2 KiB) Viewed 12 times
QUESTION 26 Determine the mass in grams of Al2(SO4)3 that contains 3.25 x 10^22 oxygen atoms.
QUESTION 37 8 points Save Answer A chemist analyzes a compound that is 26.72% sodium, 27.92% carbon, 1.96 % hydrogen and 43.40% oxygen. Determine the empirical formula of the compound.