Page 1 of 1
D Question 12 4 pts A solution contains 0.0480 M Ca²+ and 0.0940 M Ag'. If solid Na3PO4 is added to the mixture, what is
Posted: Wed Jul 06, 2022 10:28 am
by answerhappygod
- D Question 12 4 Pts A Solution Contains 0 0480 M Ca And 0 0940 M Ag If Solid Na3po4 Is Added To The Mixture What Is 1 (25.47 KiB) Viewed 12 times
D
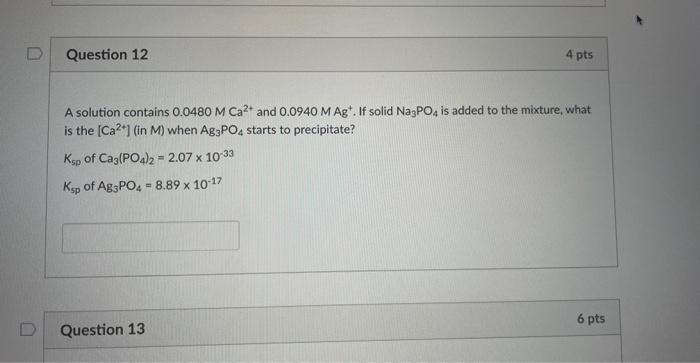
Question 12 4 pts A solution contains 0.0480 M Ca²+ and 0.0940 M Ag'. If solid Na3PO4 is added to the mixture, what is the [Ca²+] (in M) when Ag3PO4 starts to precipitate? Ksp of Ca3(PO4)2 = 2.07 x 10-33 Ksp of Ag3PO4 = 8.89 x 10-17
Question 13 6 pts