Page 1 of 1
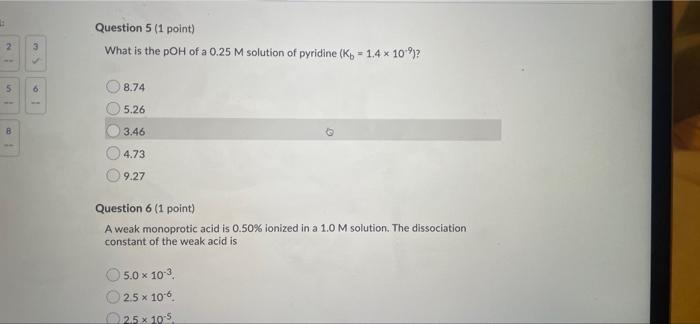
Question 5 (1 point) What is the pOH of a 0.25 M solution of pyridine (kb - 1.4 x 10-º)? 2 5 6 8.74 5.26 B 3.46 4.73 9.2
Posted: Sat Feb 26, 2022 12:00 pm
by answerhappygod
- Question 5 1 Point What Is The Poh Of A 0 25 M Solution Of Pyridine Kb 1 4 X 10 O 2 5 6 8 74 5 26 B 3 46 4 73 9 2 1 (16.93 KiB) Viewed 51 times
Question 5 (1 point) What is the pOH of a 0.25 M solution of pyridine (kb - 1.4 x 10-º)? 2 5 6 8.74 5.26 B 3.46 4.73 9.27
Question 6 (1 point) A weak monoprotic acid is 0.50% ionized in a 1.0 M solution. The dissociation constant of the weak acid is 5.0 x 103 25 x 10-6 25 x 10-5