Page 1 of 1
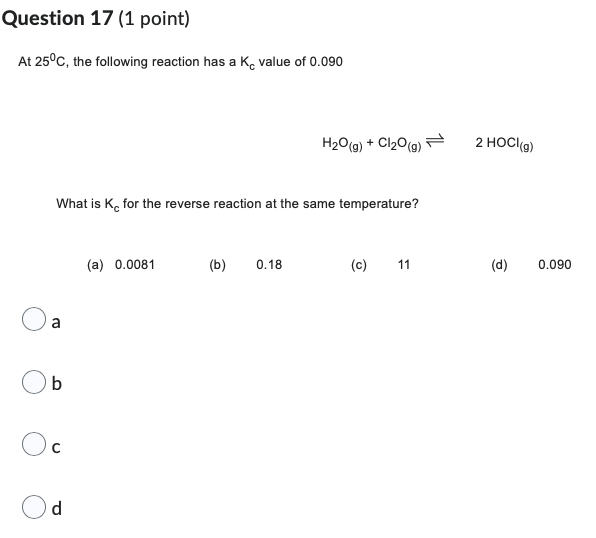
Question 17 (1 point) At 25°C, the following reaction has a K value of 0.090 H₂O(g) + Cl₂O(g) What is K, for the reverse
Posted: Thu Jun 09, 2022 8:58 am
by answerhappygod
- Question 17 1 Point At 25 C The Following Reaction Has A K Value Of 0 090 H O G Cl O G What Is K For The Reverse 1 (20.64 KiB) Viewed 54 times
Please please answer the
question as soon as possible! Will
thumbs up!
 Question
Question 17 (1 point) At 25°C, the following reaction has a K value of 0.090 H₂O(g) + Cl₂O(g) What is K, for the reverse reaction at the same temperature? (a) 0.0081 (b) 0.18 (c) 11 a b d 2 HOCI (g) (d) 0.090