Page 1 of 1
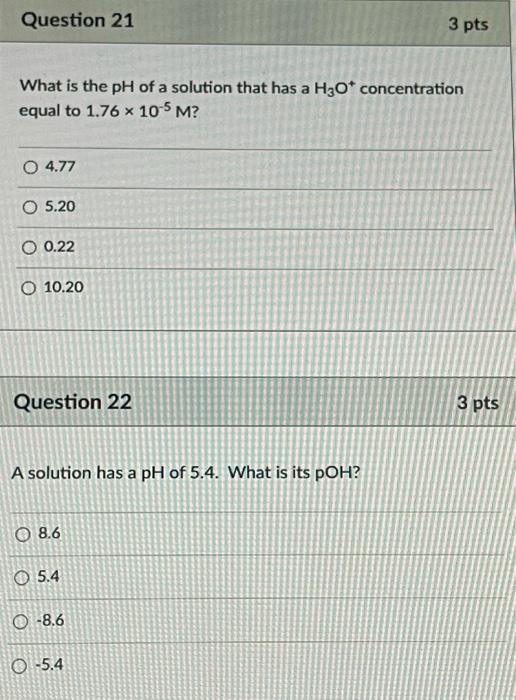
Question 21 3 pts What is the pH of a solution that has a H2O* concentration equal to 1.76 x 10-5 M? 0 4.77 O 5.20 O 0.2
Posted: Sun May 22, 2022 5:44 pm
by answerhappygod
- Question 21 3 Pts What Is The Ph Of A Solution That Has A H2o Concentration Equal To 1 76 X 10 5 M 0 4 77 O 5 20 O 0 2 1 (65.95 KiB) Viewed 10 times
Question 21 3 pts What is the pH of a solution that has a H2O* concentration equal to 1.76 x 10-5 M? 0 4.77 O 5.20 O 0.22 O 10.20
Question 22 3 pts A solution has a pH of 5.4. What is its POH? O 8.6 O 5.4 0 -8.6 0 -5.4